Element will be exhibiting at the 5th Transdermal & Intradermal Drug Delivery Systems 2018 conference on September 6-7 in Philadelphia, PA. Gain insights into our use of new technologies and latest materials and product qualification testing methods that enhance both transdermal and intradermal drug delivery.
About the event
The 2018 Transdermal & Intradermal Drug Delivery Systems conference is covering Advanced Design, Development, and Delivery of Skin-Mediated Therapies and Vaccines. This two-day intensive conference brings together leading researchers in the field to share the most recent advances in the design, formulation, and delivery of skin-mediated therapies and vaccines.
More details about the eventhere.
Why attend?
Stop by Element's booth to see how we can assist you with meeting strict demands of the global pharmaceutical sector, supporting your analytical development and product characterization needs as well as providing you with an accurate and independent assurance of safety and performance to reduce your costs.
Register today and join us in Philadelphia!
Book a Meeting Today!
Schedule a meeting with one of our Engaged Experts to discuss Element’s comprehensive range of services offered to the Pharmaceutical industry, including:
- Full CMC Product Development of both Innovative and Generic Drug Products
- Manufacture of Tox, Phase 1 & Phase 2 Clinical Batches
- Characterisation of molecule and Pre-formulation studies
- pH/Solubility and Excipients Compatibility studies
- Formulation Development studies
- Full analytical and microbiological support for the above
- Evaluation and selection of Container/Closure systems (vials and syringes)
- Development and selection of Process and key parameters
- Development of Master Batch Records
- QC release and stability testing of marketed products
- Tech Transfer & Regulatory Documentation (IND, NDA, ANDA)

Medical Device
As a comprehensive testing partner, you’ll enjoy the benefit of a single supplier source for all of your testing needs, from mechanical testing and environmental simulation to EMC and wireless device testing.
Read More

乐动LOL
发现博客文章,文章,白皮书,webinars, and advice from our world-leading testing, inspection, and certification experts.
Read More
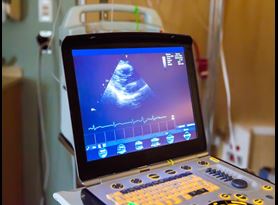
Medical Device Safety Testing
We provide medical device manufacturers with the ability to identify the appropriate safety testing standards and remove both cost and risk out of the product verification and validation process.
Read more
Element Locations
Learn more about our laboratories - where they are located; the unique capabilities they have and how they can help you solve your technical and commercial challenges.
FIND A LABORATORY