Article
Tips for Medical Device Fatigue Testing

By Engaged Expert
Calvin Chao
Fatigue testing is utilized for characterizing material properties and specimen behavior during cyclic loading. Understanding the durability and limits of the device allows medical device manufacturers to bring safe and reliable products to market.
Given the complex regulation and high risk of medical device applications, fatigue testing must be stringently performed to the appropriate stress levels, sample size, and standards according to well-defined test protocols.
How is medical device S/N fatigue testing performed?
Medical device fatigue testing focuses on the nominal stress required for failure after a number of cycles. Stress (S) is typically plotted on a log scale against the number of cycles to failure (N), also known as S/N Fatigue Testing.
During an S/N fatigue test, the first sample group is tested at high peak stress (or strain in a variation of the test) where failure occurs after a low number of cycles. Subsequent samples are tested at decreasing stress levels until a statistically significant number of samples do not fail in the specified number of cycles. The fatigue threshold is the highest stress at which a runout (non-failure) occurs.
How to Determine Sample Size
Determining the appropriate sample size is a balance between the number of tests needed for the highest level of statistical confidence and the cost of developing the test specimens. The number of samples needed depends on the standard deviation and desired levels of statistical significance. Power analysis calculators are available online to help you determine the best sample size for your application.
样品和跳动整形设备疲劳Testing
Orthopedic devices experience various forces during their use inside the body, including fatigue, torsion and wear. The number of samples needed for an orthopedic device fatigue test may vary based on device type, materials and more.
During bone plate fatigue testing, for example, six samples are often used. Four of the samples are tested to failure at the higher load levels, and two are used as runouts. If there are minor changes made to the product or process, only the runout samples may need to be retested to confirm that the changes did not impact device performance.
Sample design and failure detection methods for Intravascular Device Fatigue Testing
For intravascular device tests without a predicate, the required sample count and number of test levels are often higher than with an orthopedic device. For example, a strutted stent design might use a six or twelve specimen fixture for each test level.
To achieve better granularity on the cycles (N) and stress (S) axes, six or more test levels might be used with each six- or twelve-specimen test setup. While the number of tests might be six, the number of specimens tested could be 72. When testing multiple specimens, it is useful to incorporate a failure detection mechanism so that specimen failures can be tracked without the need for constant visual inspection.
Test coupons for intravascular device fatigue tests are often diamond-shaped for stents and valves and might be either cut directly from a device (1:1 scale) or scaled up, so they are easier to handle if device dimensions are very small.
The Element advantage
As a comprehensive medical device testing partner, you’ll enjoy the benefit of a single source supplier for all of your testing needs, from product qualification and mechanical testing to material characterization and microbiological evaluation.
Our medical device experts work diligently to help your company mitigate risk and bring safe products to patients worldwide.Contact usto discuss how we can help with your medical device fatigue testing.
Get advice for other medical device fatigue testing
How to create a test protocol for medical device fatigue testing projects
To ensure a successful fatigue test project of any size, it is critical to perform the testing in line with a clear and thorough test protocol. Engaged Expert Maciej Jakucki outlines how to structure your document, analyze requirements and define acceptance criteria in ourTest Protocol for Medical Devicesarticle.
Which medical device fatigue test system should I use?
There are different systems available for fatigue testing of medical devices. While most 21st-century fatigue testing applications leverage linear motor technology, each technology possesses unique characteristics that make it indispensable to medical device testing. OurComparison of Medical Device Fatigue Test Systemsarticle explains the difference between Servohydraulic, Servopneumatic, Single Phase and Multi-Phase Linear Motor test systems.
Do’s and Dont’s of Medical Device Mechanical Testing
For more guidance on fatigue testing and mechanical test programs for medical devices, read our article on theDo's and Don'ts of Medical Device Mechanical Testing.
View more articles aboutMore Sectors
Find related articles to you through theNucleus
making certain for nearly 190 years
More from Element

sector
Medical Device
As a comprehensive testing partner, you’ll enjoy the benefit of a single supplier source for all of your testing needs, from mechanical testing and environmental simulation to EMC and wireless device testing.
Read More
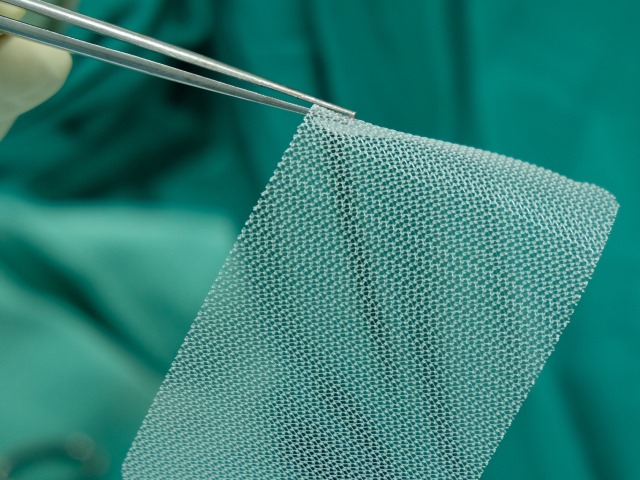
Article
Hernia Mesh Testing
Having tested surgical meshes for over 10 years, our engineering team is experienced in assessing the mechanical properties of these devices using suitable fixtures for sample gripping and loading and well-designed test protocols.
Read More

Resource
High Speed Stent and Graft Testers
Our medical device manufacturing customers are regularly challenged with short timelines to bring products to market.
read More
WHERE WE ARE
Element Locations
Learn more about our laboratories - where they are located; the unique capabilities they have and how they can help you solve your technical and commercial challenges.
FIND A LABORATORY



