文章
防止骨科器件的微动腐蚀

By Engaged Expert
Maciej Jakucki.
虽然矫形器件的植入常常为现有的医疗条件带来浮雕,但由于腐蚀而导致的二次失败,并产生腐蚀产品可以逆转阳性结果。
生物相容性测试有助于确保材料,包括不锈钢,PEEK(聚醚醚酮),聚乙烯,钛和钴铬,是植乐动娱乐官网入安全的。然而,由于这些材料随着时间的推移开始乐动娱乐官网与人类生理环境相互作用,因此相互作用可以导致植入物的促进失败或脱源,通过免疫系统抑制植入物,或者通过体内升高的金属离子水平中毒。
“微动腐蚀”在多组分设备中特别关注。当两个组分彼此接触时,循环负载会导致配合表面之间的微观运动。这种振荡运动会导致局部变形,材料去除和称为令人烦恼的转移。环境特性包括升高的温度,具有非中性pH的电解液和材料化学的差异可以在一个或两个表面上增加化学侵蚀或腐蚀的可能性。困难和腐蚀彼此的腐蚀化合物导致术语,烦恼腐蚀。
模块化接口是许多总关节置换的中央设计特征,通常是螺纹或锥形的。几何公差,表面饰面,锁定扭矩和装配力对这些连接的性能产生了重大影响。另外,许多这些配合表面是不同的材料,其可以产生可以迁移到周围组织中的腐蚀,磨损和碎屑生成的不利条件。乐动娱乐官网
Product fatigue testing can evaluate the overall mechanical performance of an implant in a laboratory environment, but additional testing and analysis are usually required to evaluate the corrosion conditions which could lead to in-vivo fracture. Such supplemental evaluations are not limited to orthopedics; electrochemical corrosion testing is often performed on other devices such as血管内行业的支架。相应的FDA指导文件概述测试时支架重叠时的测试要求,或者需要进行曲折的考虑因素时。本文将专注于肩部,膝关节和髋关节置换术设备常见的腐蚀分析。
什么是烦恼腐蚀的迹象?
- 表面变形- 外观扁平,蘑菇或其他变形的加工标记。在放大率下,甚至光滑的表面都有峰值和山谷。即使两个表面之间的标称应力可能很小,高点也会受到粗糙的触点,这可以暂时产生高应力,导致峰的变形。
- 材料转移- 从一个表面上除去材料产生粘附到另一个表面的碎片。它可以在自然界中是机械的,并且由于两个表面之间的微动而产生,通过材料(如果不同),或者由于化学还原和氧化方法可能发生,该化学降低和氧化过程腐蚀地将材料从一个表面转移到另一个表面。它也可以是这些过程的组合。
- 变色- 由于腐蚀,可能发生表面的颜色变化。腐蚀产品通常是黑色或绿色。颜色变化取决于材料和流体环境。
- 碎片生成– Changes in color of the fluid environment and visible debris presence may be observed depending on the size and quantity of the debris generated. Clear solutions may still contain significant amounts of released material in the form of metal ions, which are quantifiable by inductively coupled plasma mass spectrometry (ICP-MS) or other inspection methods.
监管考虑因素
When submitting test results to a regulatory body such as the FDA, it is often required that fretting corrosion analysis be performed to evaluate the potential for failures and ion generation. This article outlines the methods and analyses that can be performed to ensure a thorough investigative testing program that addresses fretting and corrosion concerns, as well as providing objective evidence to demonstrate safe performance.
有几种测试标准是为了帮助评估烦躁腐蚀和碎片生成的测试标准。
- ASTM F1814 - 评估模块化臀部和膝关节组件的标准指南
- ASTM F1875 – Standard Practice for Fretting Corrosion Testing of Modular Implant Interfaces: Hip Femoral Head-Bore and Cone Taper Interface
- ASTM F897 - 用于测量骨骨合成板和螺钉的微动腐蚀的标准试验方法
FDA指导文件还提供了用于测试装置疲劳性能的模块化连接,微动和腐蚀测试的建议。
你应该寻找什么?有different ways to evaluate the modular connections, some quantitative and some qualitative. What is critical is that regardless of the analysis, adequate photographic evidence is provided to support the observations (or lack thereof). Regulatory bodies and manufacturers use this evidence to evaluate whether the device has undergone sufficient testing, what the overall risk is, and how the device compares to already approved devices on the market.
测试方法
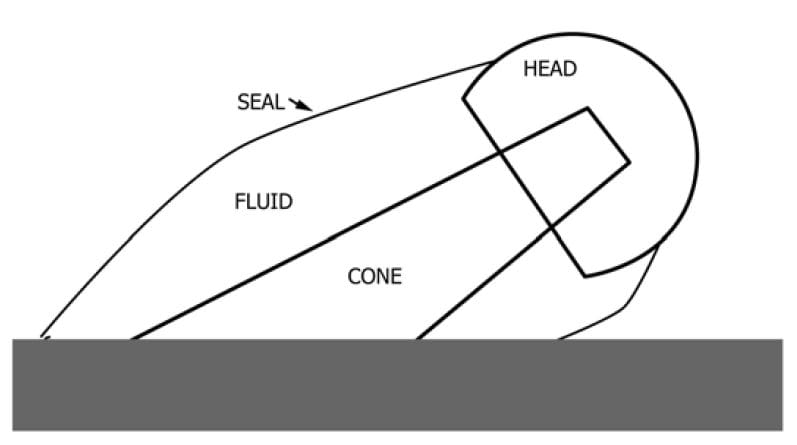
根据制造商的手术技术清洁和组装测试标本。然后在盐水溶液或牛犊血清溶液中进行机械疲劳试验以模拟体外条件。将溶液包封在感兴趣的关节周围的聚合物套管中。这是优选的,因为它限制了来自外部因素(固定装置,水泥等)的任何污染,但在所有配置中可能都不可行。测试组件可以完全暴露于溶液中,但应注意确保没有外部污染物可以进入测试室,并且夹具应设计为限制可以与试样相互作用的材料数量。乐动娱乐官网严格控制测试参数以消除任何变量至关重要。
将0.9%PBS盐水溶液加热至37℃,然后施加机械疲劳载荷,通常处于悬臂弯曲条件下。完成后,收集流体,冲洗腔室以确保捕获任何颗粒,并拆解样品。应注意保证表面不会通过工具或测试后处理损害。可以评估拆卸力以帮助确定所施加的扭矩或力是否足够以及识别是否存在锁定问题。例如,根据装载的磨损和方向,螺纹连接可以完全松动。
分析类型:
视觉和光学检查
视觉或光学检测是评估在测试期间进行微动腐蚀的效果的最常用方法。植入物用照片和一般观察记录预先测试。观察线程或锥度的初始条件将允许完整的分析。一旦拆卸样品,应执行视觉和光学检查。评估结果有几种方法。
JR Goldberg等人。开发了纸张中提到的缩放方法“A Multicenter Retrieval Study of the taper Interfaces of Modular Hip Prostheses.”此方法已被用作自2002年以来的许多评估方法的基础。HS Hothi等人撰写的另一份文件。标题为“腐蚀和烦恼的评分系统的可靠性,以及其与检索的臀部植入物的锥形模块化结的材料损失的关系”also uses this scale. Element uses a scale developed by Kevin Fricka, et al. titled“Metal-on-Metal Local Tissue Reaction Is Associated With Corrosion of the Head Taper Junction,“评估烦恼腐蚀的水平。
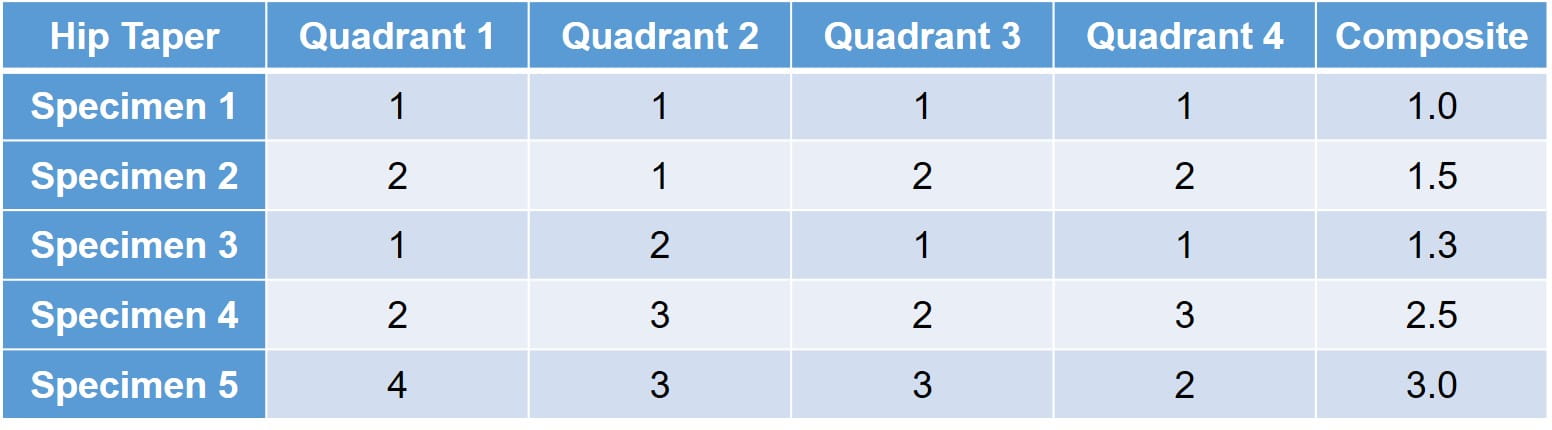
对模块化连接进行了对界面微动腐蚀的定性评估。使用1至5的等级来评估微动腐蚀,材料转移和变色。
- 1 - No visual corrosion is observed.
- 2 - 少于30%的接合锥形表面变色或沉闷。
- 3 - 大于30%的接合锥形表面变色,或者小于10%的接合锥形表面具有黑色或暗灰色碎片,蚀刻或蚀刻标记。
- 4 - 大于10%但少于50%的有关锥形表面具有黑色或沉闷的灰色碎片,蚀刻或蚀刻标记。
- 5 - Greater than 50% of the engaged taper surface has black or dull gray debris, pitting, or etch marks.
评估在表面的每个象限上进行,近侧和远侧,并且被单独得分。每个连接还接收综合评分以进一步评估植入物。理想情况下,没有观察到或最小的微量腐蚀;然而,由于加载可能不同,因此分数在不同的象限上变化很多。下面提供了一种样本矩阵:

The amount of observed fretting corrosion, material transfer and discoloration is evaluated to provide as much evidence as possible. Several examples are listed below for reference on a shoulder and a hip implant. Advanced analysis can also be performed by using scanning electron microscopy (SEM), roundness measuring machines, or white light interferometry.
Mass Loss
Another test method for evaluating the amount of wear is to perform a mass loss analysis on each of the components. Element uses a scale that has 0.00001g resolution in order to evaluate whether sample mass has changed over the course of testing. The cleaning and drying process is critical to ensure an appropriate comparison, and a control specimen (not subject to mechanical fatigue) is sometimes used to mimic the environment. If the geometry is complex, salt precipitates as well as corrosive products can also form on the surfaces which need to be cleaned away for accurate measurement. Gloves should always be worn to prevent oils from your hands from contaminating the specimens. The mass loss analysis helps validate and quantify the visual findings. Ideally, no fretting corrosion will be optically observed and will be supported by mass loss analysis data.
离子分析
The collected fluid can be analyzed for ions and wear debris using ICP-MS or other methods. Duplicates are often analyzed to observe the test repeatability.
Values below the detection limit are indicative of no or minimal ions supporting a positive test result and mitigating ion or free radical risks. The ions of interest need to be identified based on the material couple. Results can be compared to predicate data, previously-tested similar products, or to implant retrievals.
结论
腐蚀是医疗设备的重要关注。身体是腐蚀性环境,了解生理环境对材料的影响以及微观运动特性将有助于评估植入物的安全性。乐动娱乐官网本文未涵盖多种类型的电化学和腐蚀相互作用。然而,该方法概述了帮助在您的设备设计中减轻微动腐蚀风险。
遵守这些方法将有助于支持成功的监管提交和彻底了解植入物的微量腐蚀性能。在一个理想的情景中,数据将显示没有或最小的微量腐蚀,材料转移或磨损碎片,并通过质量损失和离子分析验证。在产生不利结果的情况下,与文献,检索或谓词并排测试的比较可以用于理解和预测整体植入性能。
元素优势
为市场带来创新的医疗器械技术是一种高风险的企业。这就是为什么要素努力通过为客户的医疗器械测试需求提供准确和可靠的结果,从小,启动风险到大型建立的制造商来提供绝对的测试确定。我们所订的专家在医疗器械测试的每个阶段都经历过,从测试协议开发和原型/可行性试验到测试510(k),CE标记和其他监管意见书。
作为一家全面的医疗器械测试合作伙伴,您将享受单一来源供应商的利益,可以从可行性和研发到产品开发和生产质量控制的所有测试需求。我们提供全套医疗设备测试,包括:
- 机械测试
- 产品资格认证
- 材料表征
- 微生物和化学评价
- Packaging
- EMC / EMI.
- Wireless Coexistence
- 可浸出和萃取物
- 药物
- 产品电气安全
At Element, our dedicated team of experts has many years of experience in a wide range of testing services for Class, I, II and III medical devices to help you meet medical device regulations and ensure that every aspect of your medical device product is properly tested.
有关我们烦恼腐蚀测试或其他医疗设备服务的更多信息,今天联系我们。