Article
Wireless Coexistence Testing for Medical Devices
随着无线用户的数量增加,对预期通信的干扰增长,这对无线医疗设备如无线医疗设备的适当操作构成了适当运行的风险。无线共存测试有助于减轻干扰的风险,并为全世界患者提供安全产品。
无线通信已经快速的使用ly, so the method of quantifying the risk of interference has historically been varied. In 2007, the Food and Drug Administration (FDA) issued a draft document, “Radio Frequency (RF) Wireless Technology in Medical Devices – Guidance for Industry and FDA Staff,” and followed up with the final document issuance in 2013. Section C: “Considerations for Design, Testing, and Use of Wireless Medical Devices,” outlines the FDA’s current thinking on wireless coexistence and testing recommendations. According to the FDA guidance document:
- Wireless coexistence:the ability of one wireless system to perform a task in a given shared environment where other systems (in that environment) have an ability to perform their tasks and might or might not be using the same set of rules.
无线共存测试推荐
The FDA guidance document states: “If the RF wireless medical device is expected to be used in proximity to other RF wireless in-band (i.e., the same or nearby RF frequency) sources, FDA recommends addressing such risks through testing for coexistence of the device wireless system in the presence of the number and type of in-band sources expected to be in proximity to the device. Depending upon the wireless medical device, this should also include multiple units of the subject device operating in the same vicinity, such as when patients are sitting adjacent to one another in a waiting room. Once failure modes and associated risks are identified, we recommend a justification of acceptable risk, or testing or other measures to demonstrate appropriate risk mitigation.”
我们如何测试医疗设备的无线共存
Our comprehensive approach of testing for wireless coexistence has three parts:
- 确定由于干扰导致的无线通信的故障模式和阈值:带内(共同通道和相邻通道);RF无线通信的邻近字段;乐队(80-6000 MHz)
- 满足IEE / ANSI C63.27-2017,“美国国家评估无线共存标准”,要求
- 随着新威胁出现的额外测试补充
带内测试
在带内测试确定无线通信劣化的信号 - 噪声阈值以及通信停止的阈值。带内测试的摘要提供了必要的数据,以便对收音机的行为进行更明智的风险分析,并促进从现场报告的干扰问题的故障排除。
有两个不同的设置用于带内测试,如下所示。
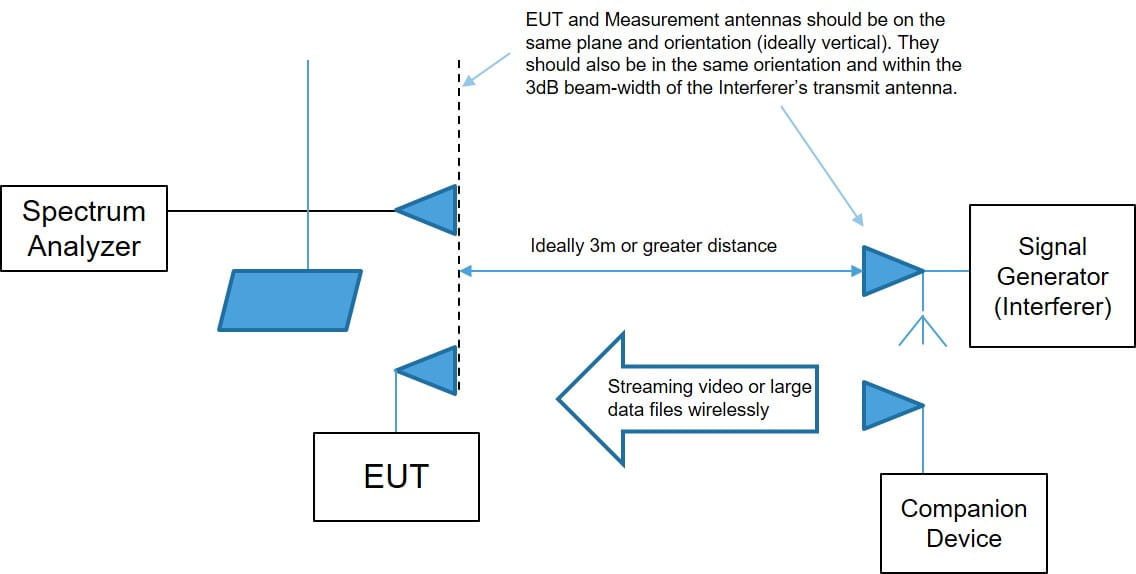
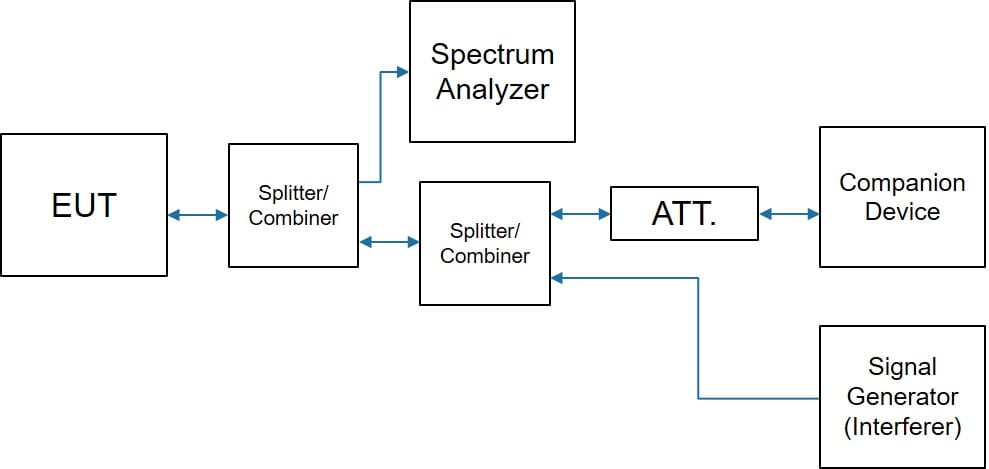
Proximity Fields
下表是测试摘要示例,提供给客户端的最小分离距离。如果在其他无线设备的接近地在其他无线设备的附近操作示例无线医疗设备,则可以使用以下表来确定设备和附近发射器之间的最小分离距离。
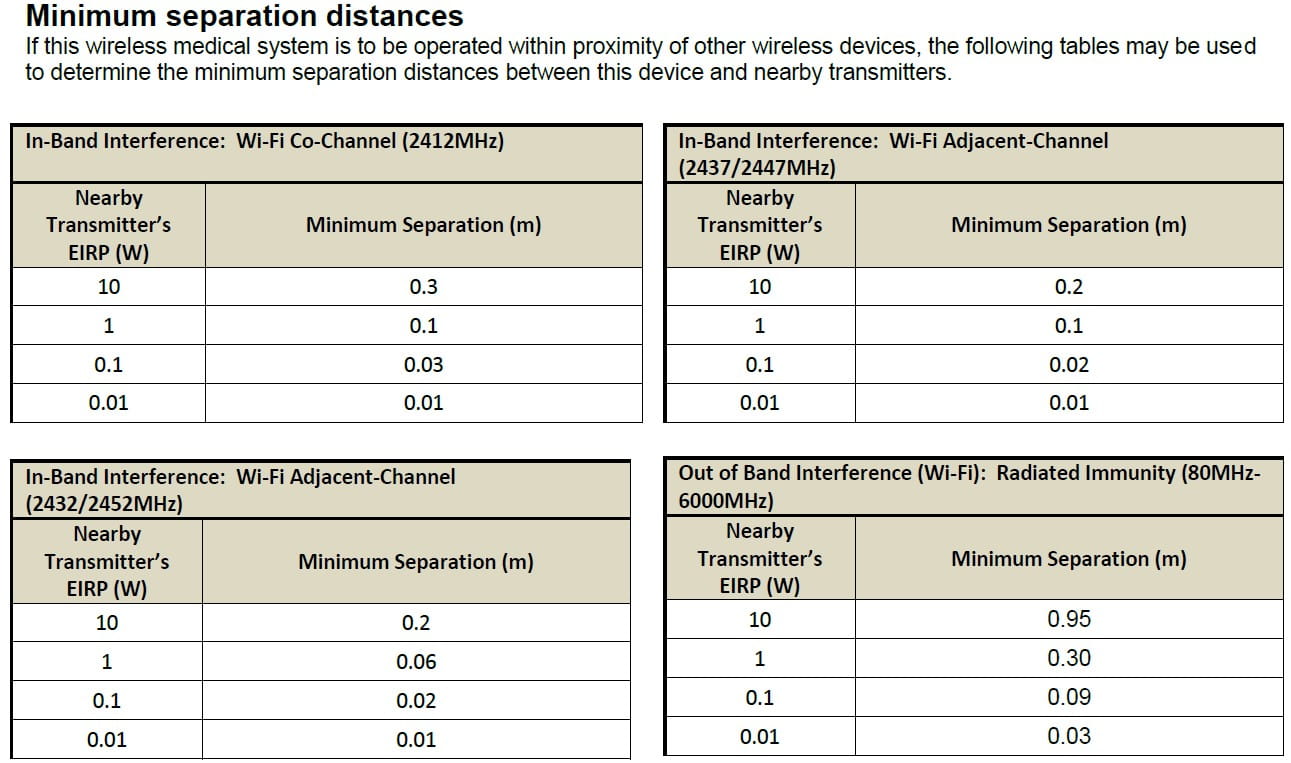
How to Mitigate In-Band Interference
认知无线电是增加频谱有效和有效使用的主要机制。它包括促进频谱共享使用的频谱感测技术。最常见的无线技术都使用认知无线电:蜂窝,Wi-Fi和蓝牙。通过实施其通信协议,本质上减少了对有效沟通的威胁。
Cellular networks have other characteristics that make them typically more robust than Wi-Fi and Bluetooth. Cellular client devices transmit from 200-700 mW compared to less than 100 mW for most unlicensed devices. The higher transmit power provides a greater signal-to-noise ratio.
Additionally, most cellular communications occur with separate transmit and receive channels compared to the transmit and receive functions occurring on the same channel for Wi-Fi. Separate transmit and receive channels reduce the risk of interference from multiple users.
How to Improve Poor Radio Performance
无线医疗器械的制造商有几种可用于无线电性能减灾的选项:
- 缩短收发器之间的链路距离。In the case of Wi-Fi radios, this could mean installing more access points. For cellular modems, it could mean selecting a different carrier with more or closer spaced cellular sites or installing a cellular repeater-signal booster on the premises.
- Change frequency bands.For Wi-Fi, there is only one 2.4 GHz frequency band, but there are four 5 GHz bands that are generally much less populated.
- 改善天线性能。Antenna gain and/or pattern may not be optimized for the intended coverage area. Changing the antenna to either a higher gain antenna or one with a more suitable pattern, can improve communications.
- 提高无线电敏感性。无意地系统噪声可能有时可能擦除无线电的输入,从而降低灵敏度。应识别噪声源,在可能的情况下,远离收音机的天线。
IEEE/ANSI C63.27 Compliance
当标准发布于2017年时,我们加强了我们的测试方法,以确保合规性。已实施以下附加要求:
必须模拟多个长期演进(LTE,4G移动通信标准)和Wi-Fi信号以供使用作干扰信号。我们使用具有多个许可证的两个向量信号发生器(VSG)来完成此操作以生成所需的信号。
含有蓝牙,Wi-Fi和数字增强无绳电信(DECT)设备的测试设备(EUT)的设备非常具体指导。
Three tiers defining the level of evaluation with tier selection based on the consequences of functional wireless performance failure.
医疗设备制造商必须在测试之前提供详细的测试计划。
To develop a robust test plan, device manufacturers need to establish how key performance indicators are determined and monitored and how medical device risk has been evaluated.
Supplemental Testing
Additional tests are occasionally performed such as electronic article surveillance and immunity to radio-frequency identification (RFID) testing.
The proliferation of radio frequency electronic article surveillance anti-theft systems (typically electro-magnetic, acousto-magnetic, radio frequency, microwave or video) is raising concerns for the increasing number of patients with active implants such as cardiac pacemakers due to potential adverse electromagnetic interference. Evaluation of these medical devices is routinely done by exposing them to electronic surveillance systems.
A new RFID immunity standard has been developed by Advancing Identification Matters (AIM), AIM 7351731 “Medical Electrical Equipment & System Electromagnetic Immunity Test for RFID Readers”, to provide guidance on how to evaluate the interaction of devices with RFID systems. Our test methods additionally follow IEC 61999-4-3 and IEC 61000-4-8 where applicable. The key challenge in this test is creating the software to produce the specific modulated signals on a vector signal generator. The signals used are described in Annex A-G of the AIM standard and range from 134.2 kHz to 2.4 GHz.
结论
如果在其他RF无线源的接近使用无线医疗设备,则可能需要无线共存测试来评估任何潜在的故障模块和相关的风险。如果由制造商决定,该制造商认为由无线技术实现的医疗功能不存在显着的风险,则可以使决定不测试无线共存。但是,制造商仍可决定执行测试以评估性能。
As more wireless medical devices enter the product development cycle, wireless coexistence testing is one of the many recommended tests that provides pertinent information for regulatory submissions. If you would like assistance with your device’s wireless coexistence testing, please contact us to discuss how we can help.
元素offers the broadest scope of medical device testing, so if you are looking for additional device testing services such as mechanical testing, package testing, microbiological testing, or accelerated shelf life testing,contact our experts today.
查看更多文章More Sectors
Find related articles to you through theNucleus
making certain for nearly 190 years
More from Element
医疗设备无线电测试
我们的医疗监管事务专家可以识别适当的无线电测试标准和认证,帮助在正式测试阶段中消除医疗产品核查和验证的成本和风险。乐动体育软件最新版
Read more

医疗设备
As a comprehensive testing partner, you’ll enjoy the benefit of a single supplier source for all of your testing needs, from mechanical testing and environmental simulation to EMC and wireless device testing.
Read More
医疗设备Safety Testing
We provide medical device manufacturers with the ability to identify the appropriate safety testing standards and remove both cost and risk out of the product verification and validation process.
Read more

EMC Testing
Find out about Element's comprehensive range of EMC service and testing capabilities in the UK and USA.
Read More