文章
ASTM F2582:测试髋关节假体的冲击
In total hip arthroplasty (THA), cumulative advances in wear rate reduction have resulted in a decline in particle-induced osteolysis, and wear-related revisions. With this decline, hip instability and mechanical loosening have become the leading reasons for revision surgery.[1]然而,错位及其不可预测性的水平仍然是THA的重大挑战。
Inferring any predisposing patient factors is difficult with any significant statistical power, even with large sample size clinical data, due to the large number of variables. Although rare well-controlled single-surgeon long-term studies exist, utilizing few changes in implant and surgical technique, the data provide benefits in terms of statistical power but lack the scope of changing key variables of interest.[2]
Hip prostheses challenges
由设备制造商控制的贡献可能会影响不稳定和错位。除了外科技术,患者处置和患者活动因素外,变量可包括头部尺寸,衬里唇形倒角角,杯插入深度,杯衬里偏移,或股杆偏移。此列表是说明性的,而不是详尽无遗,以显示有多少变量有助于髋关节不稳定。
Element approaches the renewed focus on these challenges by leveraging its diverse and extensive history of mechanical testing experience with industry-leading biomedical simulation test equipment, as well as a range of custom designed test equipment. With this capability, our orthopedic device experts help customers understand the complex mechanisms of their hip systems for development as well as device clearance through the FDA and other regulatory bodies.
我们对抗测试的方法
Within the past 10-15 years, standardized mechanical test methods for hip impingement were developed to more closely replicate clinical failure modes. While there are many test methods for hip devices, the focus of this article is on ASTM F2582-Standard Test Method for Impingement of Acetabular Prostheses as it pertains to single-piece acetabular prostheses, modular prostheses and constrained prostheses manufactured from metallic, ceramic or polymeric materials.
The ASTM F2582 test method involves a displacement controlled test in which the acetabular liner is subject to repeated impingement from the femoral stem, while simultaneously undergoing physiologically relevant articulation and loading. From the initial reference position in which the stem is in contact with the liner, the system is articulated 0°-5° in abduction, 0°-10° in extension and -5°-5° in internal/external rotation. Figure 1 graphically illustrates the phase alignment of the motions.
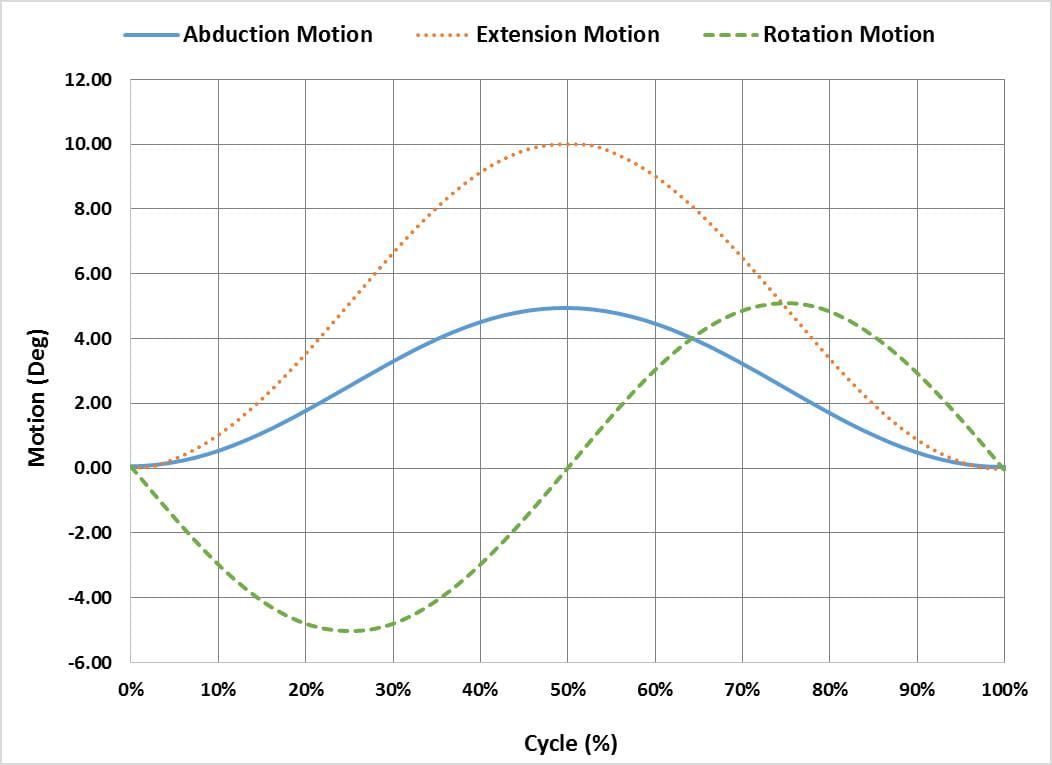
In addition to the motions, a constant 600N joint reaction force is applied. The force should be applied orthogonal to the entry plane of the liner, as shown in Figure 2 but has also successfully been applied through the stem as the liner articulates, as shown in Figure 3.
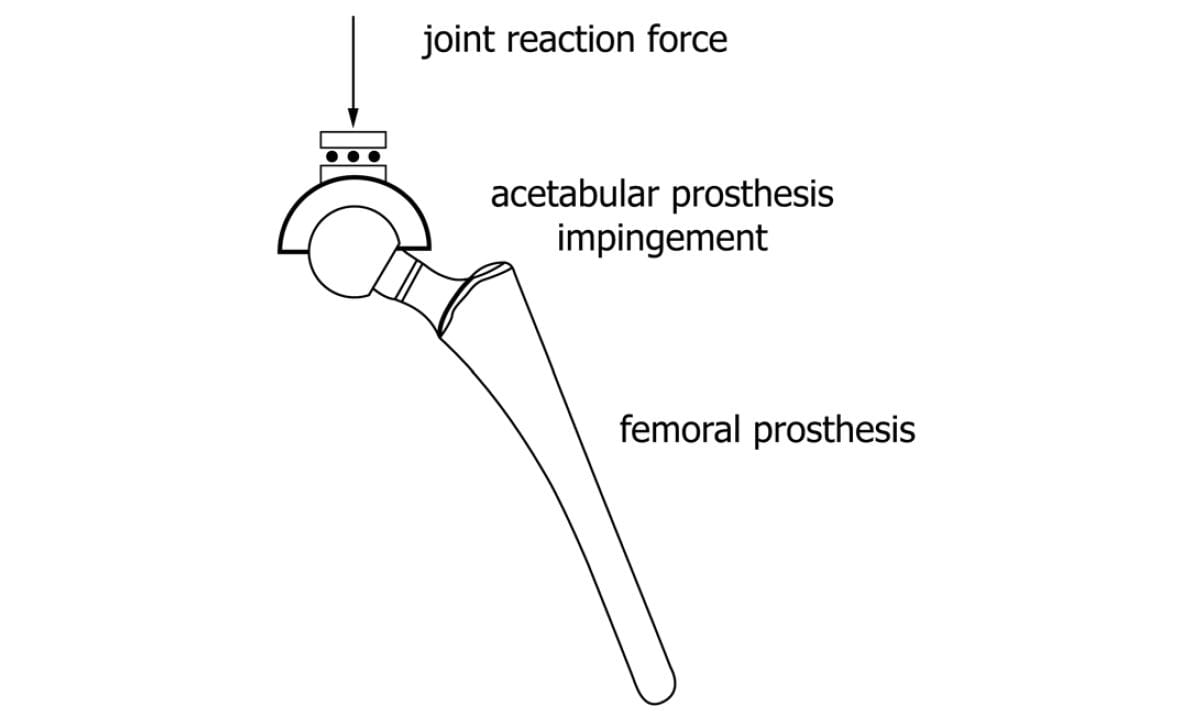
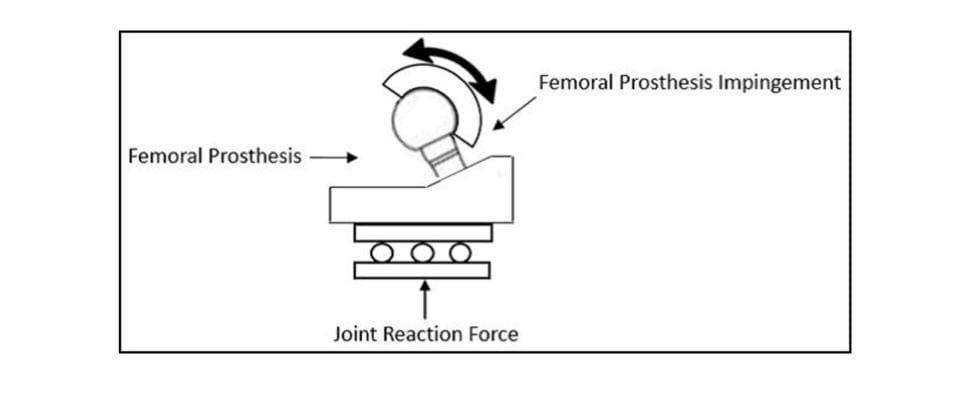
Preparing specimens for ASTM F2582 testing
The oxidation of the finished and packaged ultra-high molecular weight polyethylene (UHMWPE) liners and subsequent degradation of chemical and mechanical properties is measured in years. Preconditioning according to ASTM F2003 - Practice for Accelerated Aging of UHMWPE after Gamma Irradiation in Air aids in accounting for any degradation of the liners. The method involves immersing the liners in a pure oxygen environment at elevated pressure and temperature for two weeks.
Afterward, the surface geometry of the unworn acetabular liners is measured. A second geometry measurement is taken post-test to calculate the volume change due to impingement. Typically these measurements are taken with a structured blue light or CT scan. Solid models are created, and a scan-to-scan comparison is performed to determine volume change.
最后,根据ISO 14242-2进行预处理,该标准化方法是以间隔清洁和称重衬里的标准化方法,同时在37℃下浸泡在牛犊血清溶液中。衬垫在几天内吸收流体,并用该过程重复称重,直至建立稳定的流体吸附速率。该步骤中的最终重量测量用作衬里的预测试质量,然后将衬垫装配到测试夹具中以开始测试。
冲击测试方法
通常,该测试涉及施加600N的恒定关节反作用力,并每次执行每次200k周期的五个间隔。股骨杆在绑架运动中撞击衬垫边缘,同时也旋转屈曲 - 延伸和内部旋转。在间隔之间,调节或重置冲击位置,以确保在整个循环中发生杆和衬里之间的接触。测试通常在牛犊血清的溶液中以37℃的频率在1-2Hz的频率下进行,最高3Hz。在周期性质量损失分析的间隔之间移除衬里,尽管可以省略该过程,如在挑战和建议部分进一步讨论的那样。
共有七个标本通常用于评估每ASTM F2582的髋关节假体。三个标本称为“冲击标本”,经历关节反作用力和展示 - 进一步,屈曲 - 延伸和内部旋转。另外三种样本用作铰接控制,并且在相同的条件下测试,除了它们被定位成使得在衬里上的股骨杆的冲击永远不会发生。最终标本充当负载的控制样品,并且仅经历联合反应力,允许液体吸收的去耦合。类似地,铰接控制样品允许由于磨损引起的质量损失撞击而导致的质量损失的去耦合。
冲击标本固定
在大多数情况下,髋臼的shell is carefully cemented inPMMA bone cement在delrin或316ss夹具中。股骨杆是修改的全髋部杆,以适合于专门设计成适合测试模拟器的机械加工耳机,同时仍然保持最终部分的所有临界几何形状。在任何一种情况下,需要仔细地加工股骨头以使冲击位置与所需的最坏情况取向对准。
ASTM F2582 challenges and recommendations
There are a few parts of the standard that require knowledge of the purpose for conducting the test and careful stewardship over how the test is carried out. Additionally, it is important to know what the test can and cannot do. We will discuss a few areas that require extra attention and consideration.
One challenge is the adjustment of the abduction angle during the interval inspection periods. During this step, the stem is rotated in abduction motion until it impinges on the liner, and this location becomes the new starting point for abduction motion. Adjusting the device on the test frame presents challenges due to the small test components and curved surface features, as well as the relatively large range of abduction motion that can impede access to the components. Careful visual inspection and adjustment of the device are crucial. One possibility to make this adjustment more objective would be to rotate the stem in abduction motion until a pre-defined moment is achieved. The rotation could be performed with a 6-axis load cell so that the precise starting point could be located at each interval.
另一个令人担忧的领域是测试期间达到的质量损失相对较小。这可以是每毫升百万次循环的每毫克的顺序。在试图纠正流体吸收时或当由于冲击引起的质量损失磨损时,在分离由于磨损时,进一步损失了大众损失。对于仅三个冲击样品和三个铰接对照样品的样本尺寸,这些两组之间的变化有时是不可检测的。
Furthermore, if the liners are removed from their shell every 200k cycles for mass loss analysis, any small changes that may be present could be obscured. Many times the intermittent removal and mass analysis of the liners is omitted in favor of a simple pre- and post-test measurement. Another reason for omitting liner removal is that a trend of mass loss due to impingement is not as important as the total mass loss upon test completion. Even so, mass loss from wear is rigorously tested separately per ISO 14242 or similar standards.
If interval mass analysis is to be performed, one successful technique for the removal of the liners from shells is the utilization of liquid nitrogen. Due to the dissimilar thermal expansion of the polyethylene liner and the titanium shell, exposing the acetabular components to liquid nitrogen allows for easy removal with no damage to the liner or the locking mechanism. This method works so well that the liner can be removed from the shell with liquid nitrogen without the use of any other tools to pry or force the liner.
最后,ASTM F2582定义的故障标准缺乏可测量的细节,以客观地确定衬垫性能。除此之外,标准将失败定义为“任何假体组分的总变形”。定义是暧昧的且难以持续应用,因为每个系统在冲击部位呈现永久衬垫变形。而且,在一些系统中,存在金属上金属接触,其可以产生大量的苛刻颗粒。虽然这不是在标准中解决的,但很容易看出该领域如何损坏。此外,故障标准在对质量损失分析或体积差测量方面没有说明任何内容以及如何解释这些结果。这些测量值易于报告,但对这些结果的重要性的解释需要进一步分析。
Conclusion
As impingement of the stem and liner is imminent in-vivo, it is important to characterize and understand how the hip prosthesis will perform in this condition. The ASTM F2582 method does not purport to recreate in-vivo conditions accurately. It does, however, provide a standardized means for assessing the functional performance of an acetabular prosthesis under laboratory conditions. Even without clear failure definitions, the test method is useful for systematically study varying prosthesis design considerations. Depending on various goals, investigators may choose to deviate from the test method to look at other failure modes or test parameters in more detail.
Reference:
[1]Bozic, Kevin J., et al. "The epidemiology of revision total hip arthroplasty in the United States." JBJS 91.1 (2009): 128-133.
[2]Brown, Thomas D et al. “Impingement and dislocation in total hip arthroplasty: mechanisms and consequences.” The Iowa orthopedic journal vol. 34 (2014): 1-15.
ASTM F2582-14 Standard Test Method for Impingement of Acetabular Prostheses
The Element advantage
Our Engaged Experts excel at orthopedic device testing and have worked with many challenging device designs and test setups over the last several decades. We strive to meet all your medical device testing needs in the most expedient, efficient and responsive way.Contact us讨论如何帮助您的测试项目。
Find related articles to you through the核
Get white papers, updates and event invites
Subscribe to content updates
近190年的肯定
More from Element

Orthopedic Implant Testing
作为骨科植入性测试的全球领导者,元素在评估髋关节替代品,膝关节假体,脊髓装置和许多其他植入物方面具有多年的经验。
阅读更多

Preventing Fretting Corrosion of Orthopedic Devices
The human body is a corrosive environment that can compromise the performance of orthopedic devices. The test methods outlined in this article aid in preventing fretting corrosion in your device designs.
阅读更多

医疗设备的测试协议
议定书和计划将减轻您的风险,防止混淆,设置明确的期望,并保留未来参考和使用的必要信息。
阅读更多
Biomechanical Testing of Cadaveric Specimen
Cadaveric biomechanical testing is one of the most beneficial forms of mechanical testing in the medical industry. Our medical device testing experts explain the advantages and challenges of testing with cadaveric specimens.
阅读更多

