Article
股骨膝关节组件的疲劳试验
Total knee replacements undergo a significant battery of tests designed to measure fatigue, constraint, stress distribution, wear, interlock strength and a variety of other forces. Fatigue testing provides understanding of a component’s material properties and behavior during cyclic loading. This article will focus on fatigue testing of femoral knee components in both a closed and open condition.
Total knee replacement (TKR), or total knee arthroplasty (TKA), is a surgical procedure used to replace the articulating components of the femur and the tibia to eliminate pain due to damaged bone surfaces. The weight-bearing cartilage covering on the bones can get worn out or damaged over time, usually due to different types of arthritis such as:
- Osteoarthritis – a degenerative joint disease which breaks down joint cartilage
- 类风湿性关节炎-一种导致滑膜囊发炎导致滑液过多的疾病
- Post-traumatic arthritis – a condition often occurring from the trauma of injury to a joint
一旦软而光滑的软骨磨损,骨头就会直接相互接触,引起剧烈疼痛。当关节试图自我修复和融合时,这也会导致额外的骨生长(刺),通常会导致额外的疼痛。在全膝关节置换术中,使用单髁或双髁植入物替换骨表面及其之间的软骨。植入物通常由三部分组成:
- Femoral component for the distal femur, typically made of Cobalt Chrome per ASTM F75 or other similar specifications
- 胫骨托盘组件,通常由钛或钴铬制成
- 聚乙烯(UHMWPE)制成的全等塑料嵌件
Test specifications and guidance documents
现有FDA指导文件和行业测试规范:
- Class II Special Controls Guidance Document: Knee Joint Patellofemorotibial and Femorotibial Metal/Polymer Porous-Coated Uncemented Prostheses; Guidance for Industry and FDA
- ASTM F2083 - Standard Specification for Knee Replacement Prosthesis
- ASTM F1800 - Standard Practice for Cyclic Fatigue Testing of Metal Tibial Tray Components of Total Knee Joint Replacements
- ASTM F1223-测定全膝关节置换约束的标准试验方法
虽然大多数膝关节置换的试验方法都有规范,但还有一些附加方法,包括尚未定义的股骨膝关节组件的疲劳试验。尽管本测试通常进行,但截至编写本文件时,还没有测试标准。ASTM正在开发一个新标准,可能在2020年发布。
As the push for implants to be thinner or more patient-specific continues, it creates a risk of fracture at the different angular junctions. Although rare, some clinical failures have been observed, so mitigating risk with the appropriate test program is crucial. Fatigue testing of femoral knee components is an important step in the validation process to ensure patient safety and device longevity.
该元件提供几种配置的股骨膝盖组件的疲劳测试。
股骨膝盖组件疲劳试验配置
关闭条件:此配置以某个弯曲角度(通常为90度)向旋转轴中心向内施加载荷。该设置模拟了不适当的植入物配合或骨丢失导致股骨组件下有间隙,并且没有完全支撑的加载条件。关节面或髁间切迹的失败是最常见的。关闭条件是目前测试的最常见配置。
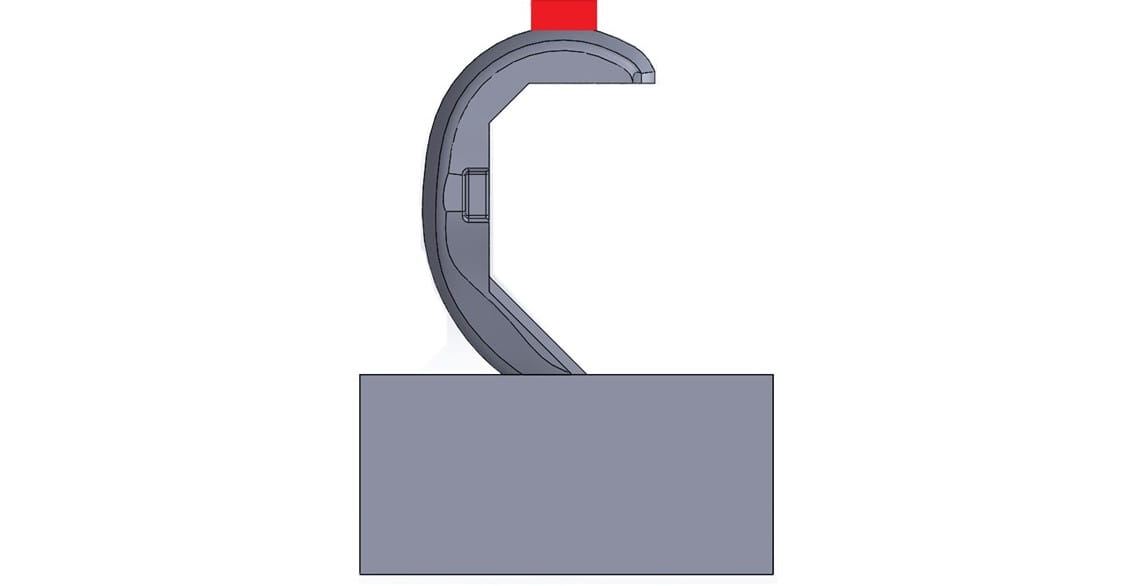

开放条件:In this configuration, the device is loaded so that it splays open the femoral component and applies high stresses to the rough surfaces on the inside of the implant. These forces may cause failure in the clinical setting due to lack of bony support on the distal femur or improper preparation of the femur during surgery. There are two ways to simulate these forces during testing:
- The femur rests on a fixture replicating the femur with either no distal bony support or mis-angled sagittal cuts. Joint compressive force is applied, forcing the femur onto the fixture and resulting in a splaying or opening load.
- 股骨的前翼缘被夹紧,钩状固定装置对后髁施加开放载荷。这种设置实质上是关闭式测试的反向加载。
这两种打开条件的测试方法都会产生从接触骨骼的粗糙表面开始的故障。
Assembly Configurations:Fatigue testing can also be performed on an assembled knee replacement, modifying the setup to include wedges, revision extensions, offset connectors, angles, or simulating minimum levels of bone support. These configurations are often tested for several reasons:
- To confirm runout to a target load level
- 疲劳试验后,测量部件拆卸前后的力
- 模块化连接的微动腐蚀评估
根据Element的经验,除非仅在一侧支撑,否则装配结构不会导致股骨髁骨折,但通常会导致组件松动或骨折后翻修延伸。最常见的配置包括在0度和体重倍数(710N)下进行测试。
对于所有这些测试配置,典型的测试参数包括:
- 载荷:体重的倍数或符合ASTM F1800 900 N的胫骨托盘悬臂要求。
- 频率:根据配置,频率范围可达15 Hz
- 负荷曲线:正弦曲线
- R比:10
- Embedding medium: bone cement or similar
- 目标周期:10000000
要素优势
正如ASTM的工作develop the test specification, our orthopedic device testing experts routinely perform and design custom femoral knee component fatigue testing programs. While most knee implants use similar angles in their femoral components, understanding the impact of the radii, contours, and stress risers due to the angles is critical in understanding femoral fatigue performance, ultimately resulting in less risk for the patient.
元素has one of the most expansive medical device testing scopes in the world, ranging from orthopedics and cardiovascular implants testing to EMC/EMI/product safety testing, and biological and packaging evaluations. We strive to meet all your medical device testing needs in the most expedient, efficient and responsive way.联系我们today.
通过网站找到与您相关的文章Nucleus
making certain for nearly 190 years
更多来自元素

骨科植入物测试
As a global leader in orthopedic implant testing, Element has years of experience in evaluating hip replacements, knee prostheses, spinal devices and many other implants.
阅读更多

Preventing Fretting Corrosion of Orthopedic Devices
人体是一个腐蚀性的环境,会影响骨科设备的性能。本文概述的测试方法有助于防止设备设计中的微动腐蚀。
Read More
尸体标本的生物力学测试
尸体生物力学测试是医疗行业最有益的力学测试形式之一。我们的医疗器械测试专家解释了尸体标本测试的优势和挑战。
Read More

三维打印种植体的评价方法
Compared to traditional materials, additive manufactured materials require qualifying and validating of the powders, structures, individual printers and overall design, creating additional steps for characterization.
Read More
