文章
E&L与Scull博士|乐动娱乐官网E&L研究的材料风险评估
Extractable and leachable testing shortens time to market and ensures patient safety by validating the quality and effectiveness of your drug product.
In this post, Element E&L expert Jim Scull, Ph.D., explains how conducting a Materials Risk Assessment (MRA) is a best practice for initiating an E&L project.
What is a Materials Risk Assessment (MRA)?
MRA是一种纸质运动,其中项目的组成部分,它们是集装箱封闭系统(CCS)或制造列车的一部分,这些组成部分是关于其预期用途和药物制剂制剂或制造条件的细节。
The purpose of conducting an MRA is multifold;
- To review all data provided by the vendor regarding the materials of construction (MOC) of the individual components, including any vendor-supplied extractables data
- To provide an assessment of the gaps between the vendor data and the data required to meet regulatory agency expectations
- 要提供一项研究设计,当执行时,将填充所识别的数据差距,从而产生完整的E&L提交包
开展MRA有几个优势:
- MRA担任“提交就绪”文件,为MOC提供全面的审查和评估,并概述了在E&L研究中可能会发现的目标化合物
- MRA不仅提供了一项研究设计,还包括其背后的理由
- MRA的结论可以确定MOC的供应商提供的数据足以满足可提取物的要求,从而无需进一步测试并允许该项目直接进入鹿角部分
我们如何在我们的MRA上开始?
Step 1:Ensure the person performing the assessment has in-depth knowledge of polymer chemistry, especially the typical additive packages used to prevent oxidation or enhance polymer performance. An example of such an additive includes slip agents applied to a polymer surface. A thorough understanding of such compound's reaction chemistries is necessary because the additive's degradation products often are seen in E&L studies. One of the main goals of a leachables study is to relate a specific leachable to its parent compound.
Step 2:确定与药物或患者直接接触的所有系统组件。一旦确定了这些,请与每个组件的供应商联系以获取所有可用的提取物信息。来自其他来源的信息,如供应商网站,技术应用文件和研究文献,也可能有所帮助。评估员也可以从事事先评估收集的个人信息图书馆。
第3步:在CCS项目的情况下,评估员接收制剂的细节,剂量方案,给药途径,预期的储存条件和估计的产品保质期。对于制造火车评估,需要流动路径,接触时间和温度以及有关任何组件灭菌方法的信息是必要的。
我有信息。我该怎么办?
Once the assessor has the above information, they conduct a thorough review relative to what E&L data is required to support submission to regulatory agencies. This step requires a good understanding of what agency reviewers expect for each particular application, either a CCS or a manufacturing train, for example.
在许多情况下,存在已知信息与提交所需的信息之间的间隙。当供应商信息过于含糊不适用或供应商研究中使用的提取条件没有产生提取轮廓时,这些差距不会产生,这将指示组件的“现实生活”使用。评估员真正在确定数据差距以及旨在完成E&L套餐的计划符合良好的研究时会产生影响。
结果(一个例子)
图1:用于含有并递送局部凝胶产物的活塞泵的示意图。(图是迈克尔·鲁贝托博士的礼貌)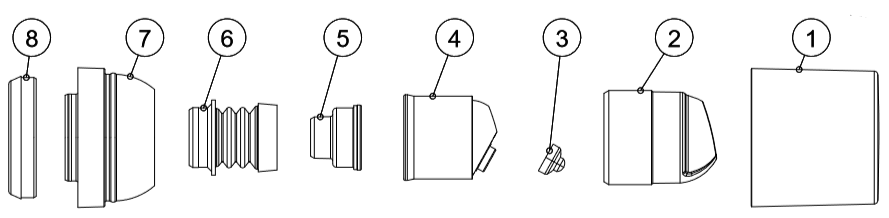
成分 |
Schematic # |
Leachables Risk |
集装箱圆 |
未示出 |
适度的风险 |
Piston round |
未示出 |
Low Risk |
Cap |
1 |
Low Risk |
执行器外部 |
2 |
Low Risk |
Membrane |
3. |
Low Risk |
执行器内在 |
4. |
Low Risk |
Reduction plug |
5. |
Low Risk |
波纹管 |
6. |
适度的风险 |
适配器戒指 |
7. |
Low Risk |
Adapter |
8. |
Low Risk |
标签 |
未示出 |
适度的风险 |
通过电子邮件通知最新博客帖子,文章,认证更新和元素新闻,乐动体育软件最新版此处更新您的订阅首选项。
For more information about extractable and leachable studies or to request a quote, please今天联系我们!!
About the Author
James R. Scull, Ph.D., Technical Director for Element Life Sciences, has more than 32 years of pharmaceutical development experience spanning all areas, from discovery support through manufacturing. His expertise includes applied analytical chemistry, toxicology, multi-dimensional separation science, extractable & leachable studies, CMC product development and oligonucleotide analysis.
Find related articles to you through the核
近190年的确定
更多来自元素
Extractables and Leachables Studies
元素的萃取物和渗滤物研究提供量身定制的解决方案,可确保患者安全和遵守行业标准。
阅读更多

药物方法开发研究
元素provides a full suite of pharmaceutical method development and research services to bring your products to market at optimal speed.
阅读更多

药物容器和包装测试
我们为聚合物和玻璃容器上的容器,容器封闭和药物包装提供专业的测试服务,以几种药典方法,包括USP,EP和JP方法。
read more

USP <761>定性和定量NMR分析
Cathy Moore, Ph.D., provides an overview of NMR spectroscopy in this free recorded webinar to help you better understand the technology, data output, and capabilities of the method.
阅读更多


